Prevalence of VA/GSM
How prevalent is Vaginal Atrophy (VA)/ Genitourinary Symptoms or Menopause (GSM)?
Vaginal atrophy (VA) is a common issue
– Significant problem in post-menopausal women.
– 50% postmenopausal women experience discomfort due to VA.1
Vaginal atrophy is undertreated
50% of post-menopausal women with genitourinary symptoms have never used any therapy for this problem.2
Patients need your help to talk about vaginal atrophy
Women want accurate medical information about vaginal atrophy, but they are more likely to want the HCP to initiate the conversation.3

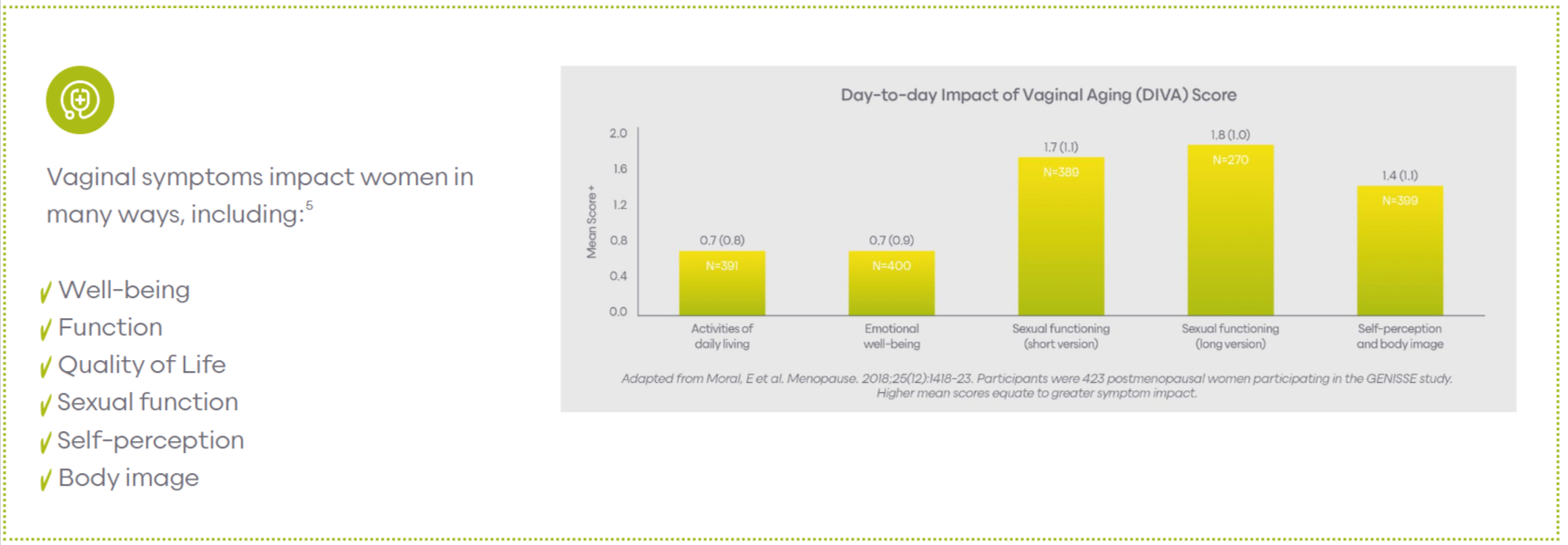
Symptoms
How do symptoms of VA impact women’s day to day life?
Common Symptoms
- Vaginal Dryness
- Dyspareunia
- Itching
- Dysuria
- Burning
Diagnosis and Treatment
- Women need prompt diagnosis and treatment.
- Early management and treatment may favour outcomes.6
- NICE guidelines suggest local treatment over systemic hormonal replacement therapy.7
- Guidelines state vaginal oestrogen should be offered to women to treat the symptoms of vaginal atrophy.
- When choosing a treatment for vaginal atrophy, the dose is important.
- The British Menopause Society WHC guideline advocates to use the lowest effective HRT dose for treating symptoms.8

- Nappi RE. and Palacios S. Climacteric. 2014;17:3-9.
- NAMS position statement. Menopause. 2020;27(9):976-92.
- Krychman M, et al. J Sex Med 2017;14:425-433.
- Palmaa F, et al. Maturitas. 2018;108:18-23
- Moral E, et al. Menopause. 2018;25(12):1418-23.
- Cagnacci A, et al. Climacteric. 2019;22(1):85-89.
- NICE. Menopause: diagnosis and management (NG23). Available from:
- https://www.nice.org.uk/guidance/ng23/chapter/recommendations.Accessed December 2022.
- British Menopause Society Women’s Health Concern Fact Sheet: HRT Benefits and Risks; Nov 2017.
IE-CH-1361(1) DOP: October 2025

